Lithium-ion batteries in the medical - pharma industry
The low weight, reduced size and power consumption of lithium-ion batteries are very beneficial for manufacturers of medical devices. This is because they are seeking to create more convenient and portable devices. Lithium ion batteries power multiple pharma products, among others, automatic external defibilators, surgical power tools, glucose monitors and infusion pumps. Because of Lithium batteries, medical devices can be become smaller and more efficiënt, without giving in on power or performance. But all these product improvements are accompanied by an increased safety risk.

For this reason, medical devices containing rechargeable lithium batteries must confirm to the IEC 62133 standard. This standard defines a number of mechanical, environmental and electrical tests that must be performed in order to ensure a product is safe. But this testing does not elimate the risk of a Lithium Ion battery fire. Several external abuse factors can cause a battery to leak, with the possibility to trigger a thermal runaway reaction.
An increased risk for patient and medical professional
While each product that carries Li-ion rechargeable batteries poses a hazard, the stakes are even higher for medical devices. The risks will be further increased when the defect of the battery leads to a nation- or globalwide recall.
It is of high importance that a recall will be implemented as fast as possible, since patients’ lives could be at stake. Imagine a worst-case scenario in which a device gets overheated, explodes and sets fire. The results could be damaging to both the patient and the medical professional.
In addition, for patients and healthcare providers, Li-ion batteries are difficult (if not possible) to remove from medical devices. All medical devices have a slim and sleek design. It prevents the ingress of elements that can have a negative effect on the performance such as liquid, dust and humidity.

Battery-powered mobile medical carts
The U.S. Food and Drug Administration (FDA) has warned the industry about fires caused by lithium batteries in mobile medical carts. Numerous reports have been received by the FDA about hospital fires and other health hazards, including explosions, fires, smoking, or overheating of equipment that required hospital evacuations. All of the incidents were associated with batteries used in mobile medical carts and their chargers. This was published in a letter on Dec. 27, 2016, on the website of the agency.
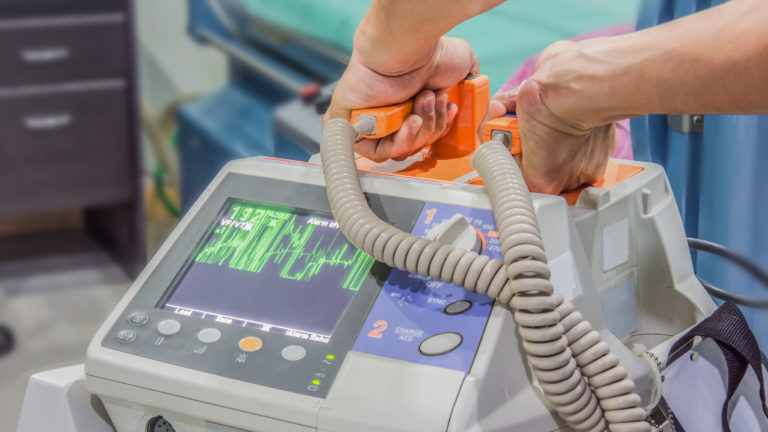
The problem only applies to the type of mobile medical cart that uses high power lithium batteries. These type of carts are capable of powering medical devices and computers (work stations) for many hours. This includes mobile carts with a therapeutic function such as medication dispensing carts, but also crash carts. The batteries are also in the carts that carry and power diagnostic medical devices (POC – point of care), barcode scanners and patient monitoring systems. Battery-powered mobile medical carts can also function as an accessory to various devices, such as ultrasound machines, colonoscopes and anesthesia machines.