The ever-growing consumption of Lithium ion batteries
The rechargable Lithium ion battery was invented in the nineties. Since then, they are widely used by many industries because of the interesting benefits they offer. Lithium ion batteries are light (Li is the lightest metal on the periodic table), very powerful and durable.
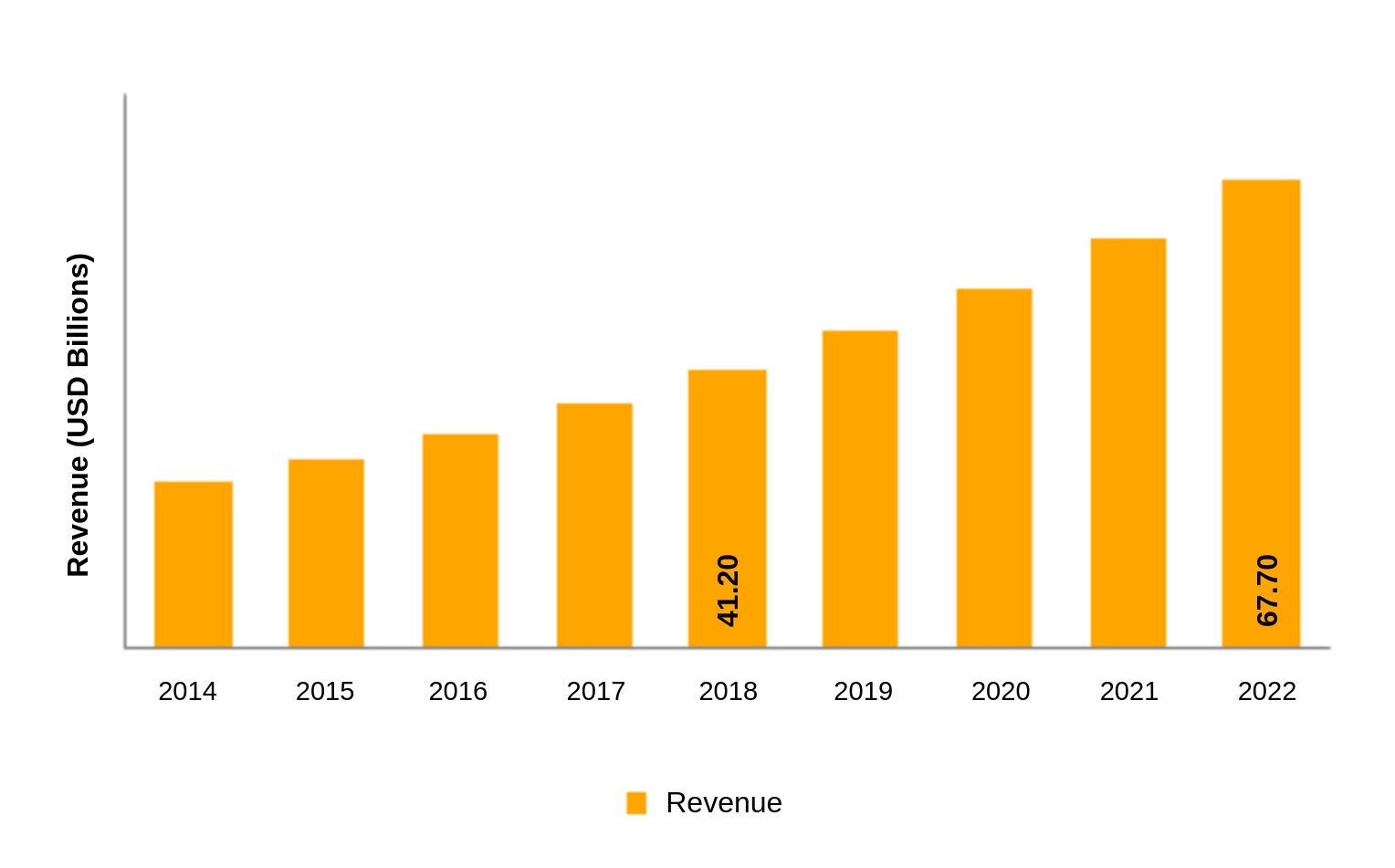
Demand for lithium ion batteries is increasing exponentially. The global lithium-ion battery market generated a substantial revenue of USD 41.20 billion in the year 2018 and is projected to grow at a CAGR of more than 10% over the forecast period.
It is our love affair with electronic gadgets, in combination with the need for renewable energy, that has led to a massive up-scale consumption. Lithium ion batteries are giving us many advantages. They have superior useable capacity, they charge fast and efficiënt, they are compact and lightweigth and have a prolonged battery life.
Lithium Ion Battery seems like the ideal solution for making our lives more comfortable and our world more durable. However, there is a major disadvantage which is that Lithium batteries can be life-threatening.
Why are these batteries so dangerous?
Part of the problem stems from what makes them so popular: They pack a lot of power for their size. But if they short-circuit they can overheat and create a chain reaction known as “thermal runaway,” a cascading effect in which they reach very high temperatures and emit smoke and toxic gasses that can fuel a fire or an explosion, especially if they’re packed tightly with other lithium batteries.
A thermal runaway can be caused by;
• Poor manufacturing, bad design
• External heating
• Over charging
• Over Discharging
• High current charging
• Structural damage
• Crush
• External short
So what is exactly happening here? Every lithium ion battery has two electrodes— there is the positively charged cathode and the negatively charged anode. They are separated from each other by a thin layer of microperferated plastic. When charging the battery, lithium ions move from the cathode through the tiny holes of the separator and an electrically conductive fluid, to the anode. The reverse happens when you discharge the battery and this reaction is what powers the electric device.

Because of bad design, manufacturing defects or several external abuse factors (described above), the separator can fail. When this happens, the anode and cathode will make contact and once they are together, the battery starts to overheat. The battery begins to hiss, bulge and leak elctrolyte. The overheating triggers an uncontrollable, explosive release of electric energy which generates smoke, flammable gas, heat (up to 600 °C to 1000 °C), fire, explosion, or a spray of flammable electrolyte. Before the cell combusts, a flare emerges briefly (~ 1 sec) The impact of the energetic release depends directly on the amount of energy stored and the type of battery.

